
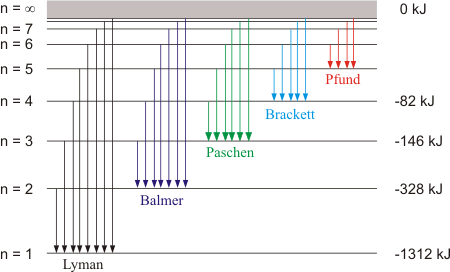
hydrogen absortion and emission spectra showing the
Emission spectrum of hydrogen

The bottom spectrum is the absorption spectrum of hydrogen.

corresponding to the atomic emission spectrum of the element Hydrogen.

Example of emission spectrum: hydrogen; (from bluegiant.phys.ksu.edu)

So Hydrogen's spectrum in the visible region looks like this.

Atomic line spectrum for hydrogen, mercury and helium.

CoreChem:Atomic Spectra and the Bohr Theory - ChemPRIME

Emission Spectra of Hydrogen Atom Under controlled conditions hydrogen gas

The emission and absortion spectrum of hydrogen in the visible
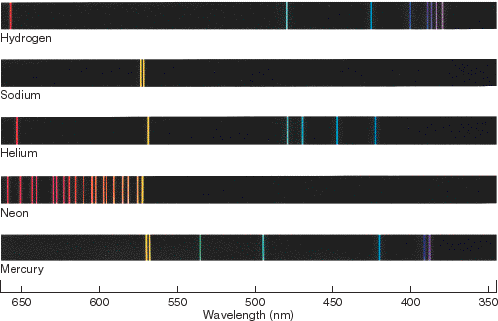
Figure 4.3 The emission spectra of some well-known elements.
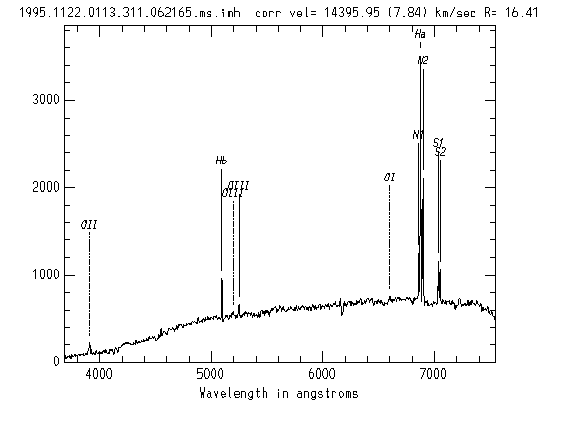
Spectrum with Labelled Emission Lines. xcsao summary page with labelled

Emission Spectra. Consider this experiment: Elecricity is passed through a
The visible spectrum of hydrogen, with its characteristic distinct lines.

Emission spectrum of hydrogen. Time: 0:58. Heated hydrogen gives off light
Hydrogen emission spectroscopy.jpg. No description
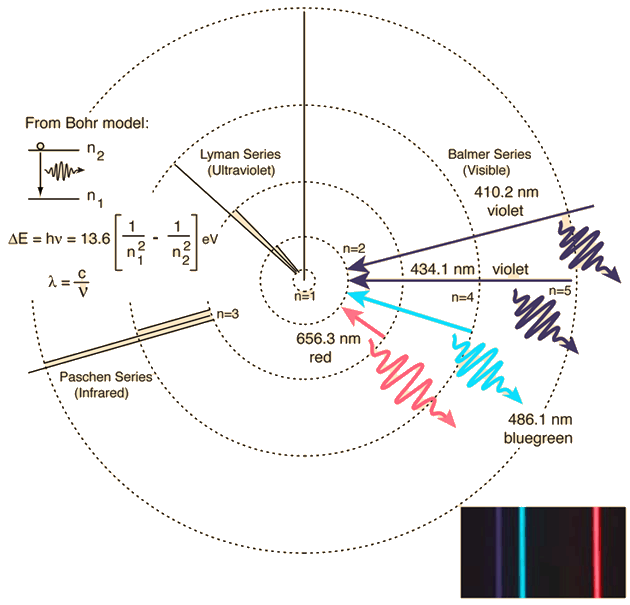
This spectrum was produced by exciting a glass tube of hydrogen

Bohr's model was able to predict the emission spectra for hydrogen but not

Hydrogen Lyman-Alpha Spectrum diagram. This map, obtained by the Japanese
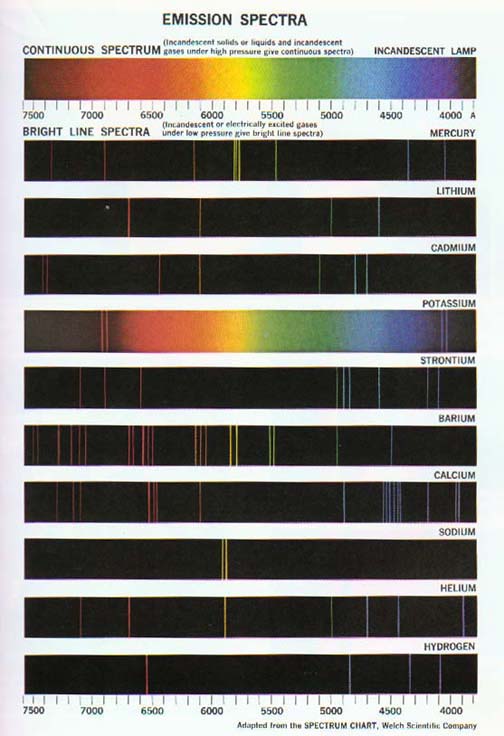
Excited hydrogen atoms produce the simplest such atomic spectra. Hydrogen